NOVEL Gene Circuits
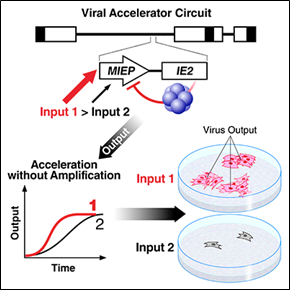
The Transcriptional Accelerator Circuit
Biological signaling circuits, like electrical circuits, face an operational tradeoff between speed and amplitude (Savageau, 1976 - pdf). That is, a faster rate of initial increase is typically obtained at the expense of a higher steady-state level. This tradeoff creates an evolutionary pressure when rapid signaling is essential but the output is cytotoxic—as with inflammatory cytokines, many viral systems and even the fever response (Roth et al., 2006).
The techniques we developed for HIV enabled our discovery of the first transcriptional accelerator circuit—an ultra-high-cooperativity negative-feedback motif that enables signaling systems (e.g. inflammatory responses) to overcome a fundamental tradeoff wherein increased speed generates higher/toxic amplitude (Teng et al. 2012). We have exploited accelerator circuits in herpesviruses as a new class of antiviral target.
Papers of note: Teng & Bolovan-Fritts et al. Cell 2012 (Linked above).
The Feedback Resistor
Animal viruses often encode positive auto-regulation of their master regulators to amplify viral gene expression. These circuits are inherently unstable if shut off, which presents a dilemma for viruses that maintain latent or dormant states. Studying HIV, we discovered a dissipative ‘feedback resistor’ circuit, where the master regulator (e.g. the HIV Tat protein) is enzymatically interconverted between active and inactive forms, transforming an unstable positive-feedback circuit into a system where the off state is stable and generates transient pulses of expression. Feedback-resistor circuits may offer a paradigm for controlling feedback and stability in diverse systems.
Papers of note: Weinberger & Shenk PLoS Biology 2007.