therapeutics THAT CO-ADAPT to CIRCUMVENT resistance
Pathogens evolve resistance to therapeutics, requiring a continual flow of new therapeutics to be developed.
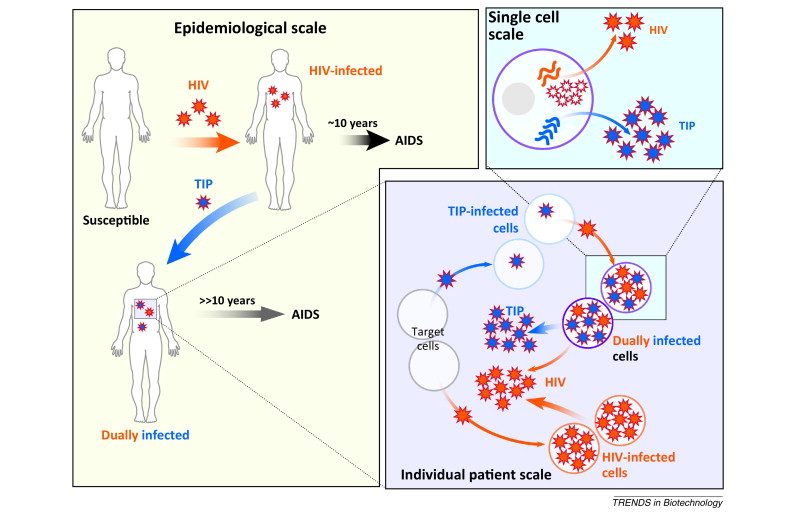
We proposed that it might be possible to develop interventions called Therapeutic Interfering Particles, or TIPs, that harness evolution and co-adapt with pathogens, to circumvent resistance. (see [Metzger et al. 2011] and [Weinberger et al. 2003])
TIPs are a type of molecular parasite of viruses based on the natural phenomenon of Defective Interfering Particles (DIPs) – viral deletion mutants that must compete with the wild-type virus for replication/packaging resources within the infected cell. Consequently, DIPs acts as ‘cheaters’ and deprive wild-type viruses of critical replication machinery, reducing wild-type virus production. DIPs arise spontaneously and transmit through human populations (Aaskov et al. 2005).
In contrast to DIPs, TIPs are engineered to have an in vivo basic reproductive ratio (R0) that is greater than 1 (R0>1).
We theoretically predicted determinants required for TIP co-adaptation (Rouzine and Weinberger, 2013) and we continue to be interested in understanding molecular determinants for co-adaptation both theoretically and experimentally.
TIP-based therapies are attractive candidates for population-wide infectious-disease control, especially in resource-limited settings (Notton et al., 2014).